AG Cardiovascular Systems
Group member
Prof. Dr. med. Andreas Wree
Dr. rer. nat. Jonas Keiler
Scientific profile
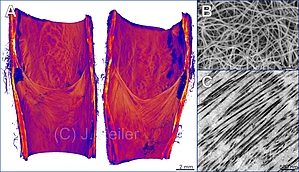
Morphological and histological studies on the human femoral vein for the development of venous valve implants
The development of a reliable long-life implant to therapy chronic venous insufficiency based on insufficient venous valves is the one of the aims of the RESPONSE research consortium. The requirements for a percutaneous applied minimally invasive venous valve implant has to be more precise considering geometrical, mechanical and morphological parameters. We therefore re-investigate morphological and histological aspects of human venous valves with a broad spectrum of imaging techniques such a x-ray microtomography and electron microscopy.
Topographical and morphological characterization of lead-vessel-interactions for the lead optimization of cardiac implantable electronic devices
Leads of implantable pacemakers (PMs) and cardioverter defibrillators (ICDs) are exposed to enormous dynamic-mechanical long-time strains. In addition, their polymeric isolation materials are subject to aging processes which decrease the long-time stability of the systems. Moreover, tissue adhesion via fibrotic processes is problematic for extractability in cases of defects. Considering these challenges and based on our morphological-anatomical findings, we develop new concepts for the optimization of leads within the RESPONSE research consortium. Our aim is the topographic and morphological characterization of neointimal/fibrotic adhesion sites forming the basis for the development of coatings protective against adhesion in turn prospectively providing patients with long-life implants.
Morphometric and histostructural analysis of the aortic valve complex and its calcifications in the elderly for the optimization TAVIs
Paravalvular leakages after endovascular transcatheter aortic valve implantation (TAVI) due to mismatched implant geometry or interference by atherosclerotic plaques is highly problematic since it is linked to a significantly increased mortality. To address this issue we re-assess the morphological properties of the aortic valve complex and the topography of its calcified areas in the aortic annulus, valve leaflets and the aortic root. Our finding will help to understand the individual patient-specific requirements for an optimal artificial valve design.
Range of methods
• immunohistochemistry / histochemistry
• electron microscopy (SEM / TEM)
• confocal laser scanning microscopy
• x-ray microtomography
• virtual 3D reconstruction
• vascular corrosion casting
Cooperation
• Clinic and Polyclinic for Heart Surgery, Heart Center Rostock, Rostock University Medical Center
• Division of Cardiology, Heart Center Rostock, Clinic and Polyclinic for Internal Medicine, Rostock University Medical Center
• Division of General, Thoracic, Vascular and Transplant Surgery, Clinic and Polyclinic for Surgery, Rostock University Medical Center
• Electron Microscopic Center, Rostock University Medical Center
• Institute for Biomedical Engineering, Rostock University Medical Center
• Institute for ImplantTechnology and Biomaterials e.V.
• Zoology, Institute for Biosciences, University of Rostock
• Rudolf-Zenker-Institute for Experimental Surgery, Rostock University Medical Center
• Department of Anatomy and Cell Biology, MLU-Faculty of Medicine Halle
• Department for Tropical Medicine and Infectious Diseases, Rostock University Medical Center
• Institute of Genome Biology, Leibniz Institute for Farm Animal Biology
Contact
Prof. Dr. med. Andreas Wree
tel.: +49 (0)381 - 494 8401
fax: +49 (0)381 - 494 8402
andreas.wree{bei}med.uni-rostock.de
