AG Frintrop
Our interest
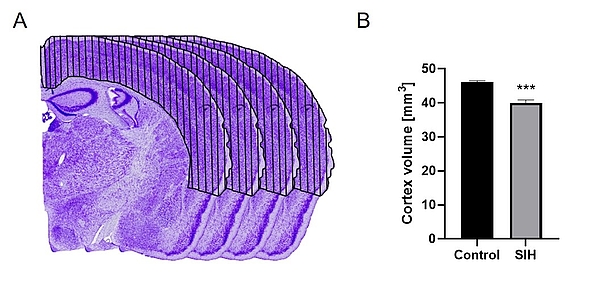
Anorexia nervosa (AN) is an eating disorder characterized by body weight loss, amenorrhea, and hyperactivity. It has the highest mortality rate of any psychiatric disorder and primarily affects young girls and women; however, increasingly, boys and men are also affected. Furthermore, brain volume loss has been associated with neuropsychological deficits in patients with AN. As with many psychiatric disorders, treatment options for AN are limited, and little is known about the underlying cellular and molecular biological mechanisms. Neurons and glial cells are becoming the focus of scientific interest.
To unravel the pathophysiology of AN, we use the murine starvation-induced hyperactivity (SIH) model, which mimics the somatic consequences of AN, such as body weight loss, hyperactivity, amenorrhea, and endocrine changes. This model involves providing an individually calculated restricted amount of food once a day and access to a running wheel. In this model, SIH mice receive 40% of their average daily food intake, calculated during an acclimatization phase, until a 25% body weight reduction is reached. This is followed by two weeks of daily food intake adjustments to mimic chronic starvation.
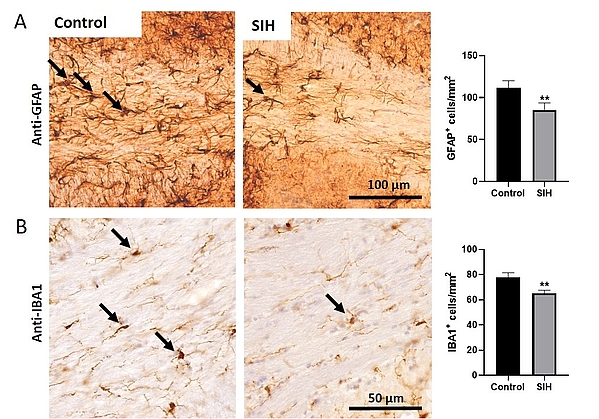
Our group focuses on investigating whether glial cell changes in the brain underlie the pathophysiology of AN. Additionally, we are interested in whether circadian changes and neurodegeneration contribute to the disorder. To address our scientific challenges, we use state-of-the-art methods such as an artificial intelligence infrared-sensory-based activity tracking system (Goblotrop), a set of behavioral analyses including the elevated plus maze and novel object recognition, and electron microscopy.
Key cooperations
- Beyer, Cordian, Trinh, Stefanie; Department of Neuroanatomy, RWTH Aachen University, Germany
- Seitz, Jochen; Clinic for Psychiatry, Psychosomatics and Psychotherapy of Childhood and Adolescence, LVR University Hospital Essen, Germany
- Kölch, Michael, Dück, Alexander, Reis, Olaf, Berger, Christoph; Department for Child and Adolescent Psychiatry and Neurology, Rostock University Medical Center, Germany
- Salomon, Ralf, Gabloffsky, Theo; Institute of Applied Microelectronics and Computer Engineering, Faculty of Computer Science and Electrical Engineering, University of Rostock, Germany
- Frank, Marcus; Medical Biology and Electron Microscopy Center, Rostock University Medical Center, Germany
- Wolkenhauer, Olaf, Baumann, Alexandra, Van Welzen, Matti; Department of Systems Biology and Bioinformatics, University of Rostock, Germany
Most important publications
- Schuster K., Staffeld A., Zimmermann A., Böge N., Lang S., Kuhla A., Frintrop L. Starvation in Mice Induces Liver Damage Associated With Autophagy. Nutrients. 2024 Apr 17;16(8):1191.
- Zimmermann A.*, Böge N.*, Schuster K., Staffeld A., Lang S., Gill S., Rupprecht H., Frintrop L. Glial cell changes in the corpus callosum in chronically-starved mice. Journal of Eating Disorders. 2023 Dec 18;11(1):227.
- Staffeld A.*, Gill S.*, Zimmermann A., Böge N., Schuster K., Lang S., Kipp M., Palme R., Frintrop L. Establishment of a murine chronic anorexia nervosa model. Cells. 2023 Jun 24;12(13):1710.
- Gabloffsky T.*, Gill S.*, Staffeld A., Salomon R., Guerra N., Joost S., Hawlitschka A., Kipp M., Frintrop L. Food Restriction in Mice Induces Food-Anticipatory Activity and Circadian-Rhythm-Related Activity Changes. Nutrients. 2022 Dec 9;14(24):5252.
- Frintrop L., Trinh S., Seitz J., Kipp M. The Role of Glial Cells in Regulating Feeding Behavior: Potential Relevance to Anorexia Nervosa. The Journal of Clinical Medicine. 2021 Dec 30;11(1):186.
Current and most recent funding
- FORUN-Programm UMR Titel: Astrozyten-Degeneration und Verhaltensdefizite in einem Modell der Anorexia nervosa
- Doktor Robert Pfleger-Stiftung Titel: Gliazell- und Verhaltensveränderung in einem murinen Modell der Anorexia nervosa
Group members
Tel.: +49 (0)381 - 494 8406
Raum: 3.50
Doktoranden*innen:
Frau M. sc. Annelie Zimmermann
Frau Julia Priebe
Frau Hanna-Sophia Henschke