Jun.-Prof. Dr. rer. hum. Sarah Joost
Kontakt:
Universitätsmedizin Rostock
Institut für Anatomie Rostock
Jun.-Prof. Dr. rer. hum. Sarah Joost
Gertrudenstraße 9
18057 Rostock
Tel.: +49 (0)381 - 494 8444
Raum 3.49
E-Mail: sarah.joost{bei}med.uni-rostock.de
Link zur Arbeitsgruppe:AG Joost
Forschungsschwerpunkte/Scientific profile
Regional heterogeneity of human meninges
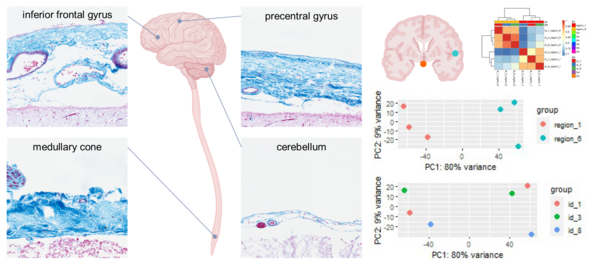
The central nervous system is surrounded by three layers of meninges: The outermost robust dura mater, the innermost delicate pia mater, and the arachnoid mater which is spanning the subarachnoid space. The meninges are large and complex structures that are, according to recent studies, involved in the recruitment of immune cells, but regional heterogeneity of human meninges has never been systematically addressed. We hypothesize that the meninges, especially the arachnoid mater, show region-specific heterogeneity in morphology and gene expression.
We are collecting post mortem samples of human meninges from distinct reproducible regions of the brain and spinal cord. Meningeal samples are processed both for histology and RNA extraction. Histologic samples are used for morphological analysis and RNA samples are used for gene expression studies by quantitative real-time PCR and next generation sequencing.
Working on this project:
M. sc. Elise Vankriekelsvenne
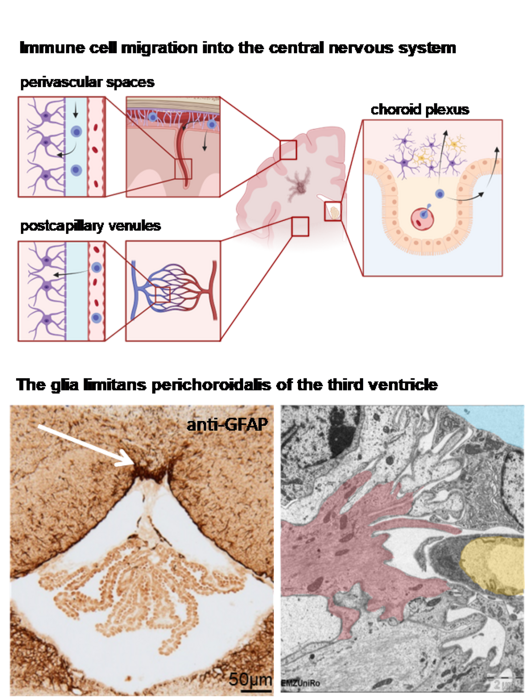
Immune cell recruitment via the glia limitans perichoroidalis
Peripheral immune cells invade the central nervous system (CNS) under neuroinflammatory conditions via different anatomical migration routes: crossing of the blood-brain-barrier at the level of postcapillary venules, egressing from meningeal blood vessels or crossing of the choroid plexus epithelium. We hypothesize that an additional migratory route exists at the attachment region of the choroid plexus that connects the choroid plexus stroma and the CNS parenchyma and that this potential migration route is protected by a highly specialized glial barrier structure, the glia limitans perichoroidalis.
We are analyzing the attachment region of the choroid plexus in the murine and human ventricle system by immunohistochemical labelling of glial and basal laminal proteins, by ultrastructural analysis by transmission electron microscopy and gene expression analysis of samples excised by laser-assisted microdissection. The relevance of the glia limitans perichoroidalis for immune cell migration is investigated in neuroinflammatory murine models.
Working on this project:
Katerina Manzhula
Louise Baumann
In cooperation with Dr. Marcus Frank, Medical Biology and Electron Microscopy Center
In cooperation with Dr. Björn Schneider, Institute of Pathology
Morphology of the murine choroid plexus
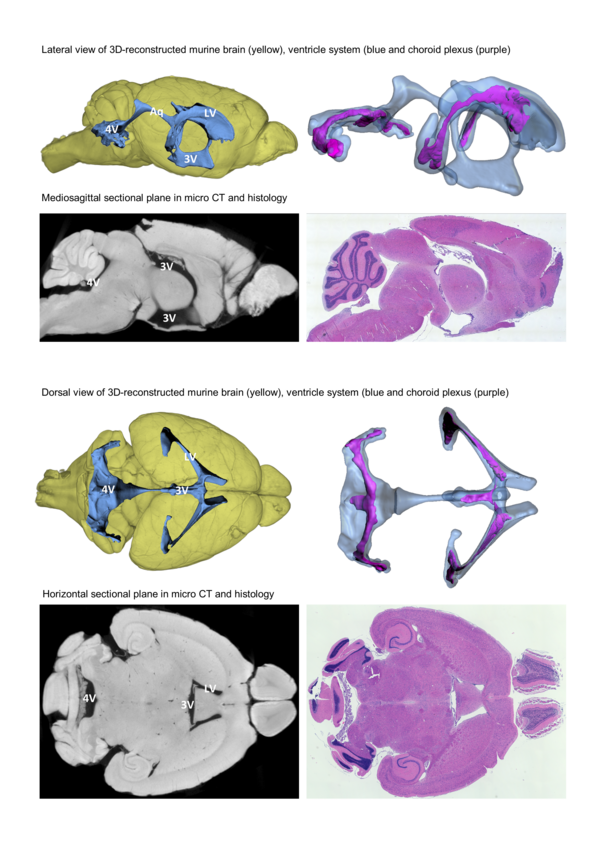
The choroid plexus is located in the ventricular system of the brain and responsible for production of the cerebrospinal fluid (CSF). It is built of a highly vascularized stroma and the tight plexus epithelium, forming the blood-CSF-barrier. Besides its role for fluid homoeostasis of the brain, the choroid plexus is discussed as a site of immune surveillance. Furthermore, it probably is an access path for activated immune cells into the central nervous system under pathological conditions.
Detailed knowledge on the three-dimensional morphology of the choroid plexus, its attachment to the brain parenchyma, blood supply routes and its spatial relationship to different spaces of the CNS (ventricular space, subarachnoid space, brain parenchyma) are the prerequisite for elucidating the anatomical route of immune cell invasion via the choroid plexus. However, in mice, the most common experimental organism, these complex structures have not yet been described in sufficient detail.
We analyze the morphology of the ventricle system and the choroid plexus by micro CT scanning and three-dimensional reconstruction supplemented by histological processing of paraffin-embedded sections.
Update: We have published our detailed description of the murine ventricle system and choroid plexus including an interactive three-dimensional model of these structures in Frontiers in Neuroanatomy:
Greiner, Theresa; Manzhula, Katerina; Baumann, Louise; Kaddatz, Hannes; Runge, Jens; Keiler, Jonas; Kipp, Markus; Joost, Sarah (2022): Morphology of the murine choroid plexus: Attachment regions and spatial relation to the subarachnoid space. In: Front. Neuroanat. 16, Artikel 1046017. DOI: 10.3389/fnana.2022.1046017.
Working on this project:
Theresa Greiner
Katerina Manzhula
Louise Baumann
Dr. Jens Runge (AG Response)
Dr. Jonas Keiler (AG Response)
The mysterious hind limb paralysis
Update: Case solved!
In our colony of RAG1-deficient mice, a surprisingly high number of animals spontaneously developed hind limb paralysis. To date, such symptoms are not described in literature for this strain. Due to the high observed incidence, we decided to clarify the cause of the symptoms.
Spines and spleens were dissected and processed for histology. Dense infiltrates of uniformly shaped cells filled both the spleen and the bone marrow cavities of vertebral bones. Furthermore, these cells infiltrated into the spinal canal at multiple sites. These cell infiltrates are further characterized by means of histology, immunohistochemistry and flow cytometry. Additionally, the structural integrity of vertebral bones is analyzed by micro CT scanning.
Update: We identified the cell infiltrates as malignant hematologic cells of the precursor B cell stage. The results have been published in Journal of Molecular Neuroscience:
Feifei, Liu; Richter, Anna; Runge, Jens; Keiler, Jonas; Hermann, Andreas; Kipp, Markus; Joost, Sarah (2022): Spontaneous Hind Limb Paralysis Due to Acute Precursor B Cell Leukemia in RAG1-deficient Mice. In: J Mol Neurosci. DOI: 10.1007/s12031-022-02025-7.
https://link.springer.com/article/10.1007/s12031-022-02025-7
Working on this project:
M. sc. Liu Feifei (AG Promyelo)
Dr. Anna Richter (Institute for Hematology and Oncology Rostock)
Dr. Jens Runge (AG Response)
Morphological changes in spine and spleen of paralysis-affected RAG1-deficient mice
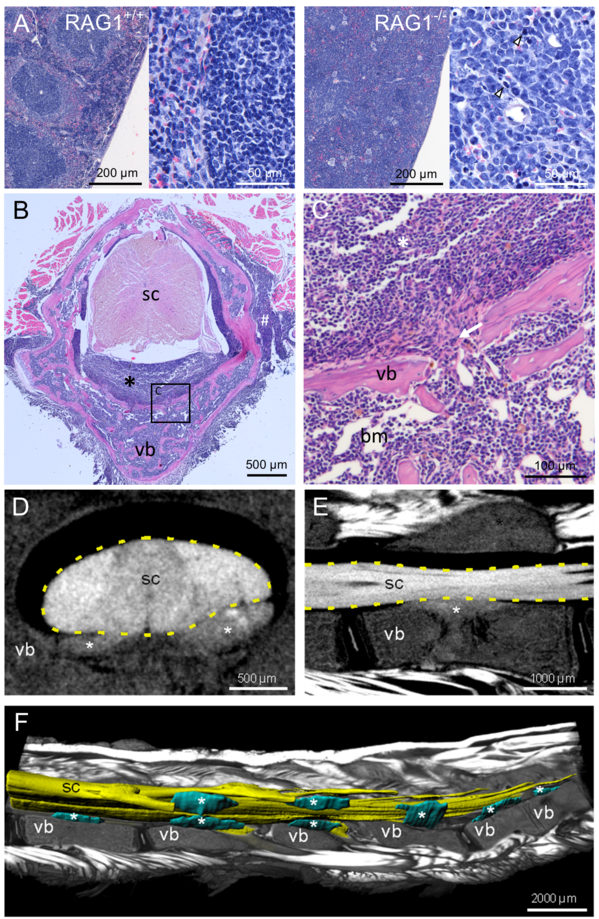
A Hematoxylin-eosine staining of spleen (paraffin) sections of wildtype controls and RAG1-deficient mice with hind limb paralysis (arrowheads highlight mitotic figures) B Hematoxylin-eosine staining of decalcified spine sections from RAG1-deficient mice with hind limb paralysis reveal cellular infiltrates (asterisk) in the spinal canal between vertebral bone (vb) and spinal cord (sc). C Enlarged view of B (square) showing discontinuities (arrow) of the vertebral bone (vb), connecting bone marrow (bm) and infiltrates (asterisk) in the spinal canal. D Virtual cross section and E parasagittal section through the spinal cord (sc) and vertebral bone (vb) based on micro-CT showing the cellular infiltrates (asterisks) in the spinal canal. F Ventro-lateral view of the lumbar spine with parasagittal section through the vertebrae and muscles (volume rendering) to expose the 3D-reconstructed (surface rendering) spinal cord (sc) and cellular infiltrates (asterisks).
Publikationen (Auswahl ab 2010)
- Greiner T, Manzhula K, Baumann L, Kaddatz H, Runge J, Keiler J, Kipp M, Joost S (2022) Morphology of the murine choroid plexus: Attachment regions and spatial relation to the subarachnoid space. Front. Neuroanat. 2022, 16.
- Behrangi N, Heinig L, Frintrop L, Santrau E, Kurth J, Krause B, Atanasova D, Clarner T, Fragoulis A, Joksch M, Rudolf H, Meuth SG, Joost S, Kipp M (2022) Siponimod ameliorates metabolic oligodendrocyte injury via the sphingosine-1 phosphate receptor 5. PNAS 2022, 119:e2204509119.
- Wittekindt M, Kaddatz H, Joost S, Staffeld A, Bitar Y, Kipp M, Frintrop L (2022) Different Methods for Evaluating Microglial Activation Using Anti-Ionized Calcium-Binding Adaptor Protein-1 Immunohistochemistry in the Cuprizone Model. Cells. 2022; 11(11):1723.
- Feifei L, Richter A, Runge J, Keiler J, Hermann A, Kipp M, Joost S (2022) Spontaneous Hind Limb Paralysis Due to Acute Precursor B Cell Leukemia in RAG1-deficient Mice. J Mol Neurosci. 2022 May 18.
- Vankriekelsvenne E, Chrzanowski U, Manzhula K, Greiner T, Wree A, Hawlitschka A, Llovera G, Zhan J, Joost S, Schmitz C, Ponsaerts P, Amor S, Nutma E, Kipp M, Kaddatz H (2022) Transmembrane protein 119 is neither a specific nor a reliable marker for microglia. Glia. 2022 Jun;70(6):1170-1190.
- Joost S, Schweiger F, Pfeiffer F, Ertl C, Keiler J, Frank M, Kipp M (2022) Cuprizone intoxication results in myelin vacuole formation. Front Cell Neurosci. 2022 Feb 18;16:709596.
- Kaddatz H, Joost S, Nedelcu J, Chrzanowski U, Schmitz C, Gingele S, Gudi V, Stangel M, Zhan J, Santrau E, Greiner T, Frenz J, Müller-Hilke B, Müller M, Amor S, van der Valk P, Kipp M (2021) Cuprizone-induced demyelination triggers a CD8-pronounced T cell recruitment. Glia. 2021 Apr;69(4):925-942.
- Rohr SO, Greiner T, Joost S, Amor S, van der Valk P, Schmitz C, Kipp M (2020) Aquaporin-4 Expression during Toxic and Autoimmune Demyelination. Cells. 2020 Sep 28;9(10):2187.
- Zhan J, Mann T, Joost S, Behrangi N, Frank M, Kipp M (2020) The Cuprizone Model: Dos and Do Nots. Cells. 2020 Mar 31;9(4):843.
- Zhan J, Yakimov V, Rühling S, Fischbach F, Nikolova E, Joost S, Kaddatz H, Greiner T, Frenz J, Holzmann C, Kipp M (2019) High Speed Ventral Plane Videography as a Convenient Tool to Quantify Motor Deficits during Pre-Clinical Experimental Autoimmune Encephalomyelitis. Cells. 2019 Nov 14;8(11).
- Joost S, Mikkat S, Wille M, Schümann A, Schmitt O (2019) Membrane protein identification in rodent brain tissue samples and acute brain slices. Cells 2019. 8(5), 423.
- Rabenstein M, Peter F, Joost S, Trilck M, Rolfs A, Frech MJ (2017): Decreased calcium flux in Niemann-Pick type C1 patient-specific iPSC-derived neurons due to higher amount of calcium-impermeable AMPA receptors. Mol Cell Neurosci. 2017 Sep; 83:27-36.
- Joost S, Kobayashi K, Wree A, Haas SJP (2017): Optimisation of murine organotypic slice culture preparation for a novel sagittal-frontal co-culture system. J Neurosci Methods. 2017 Jun 15;285:49-57.
- Pasold J, Engelmann R, Keller J, Joost S, Marshall RP, Frerich B, Müller-Hilke B (2013): High bone mass in the STR/ort mouse results from increased bone formation and impaired bone resorption and is associated with extramedullary hematopoiesis. J Bone Miner Metab. 2013 Jan;31(1):71-81.
Curriculum vitae
| seit 12/2015 | Wissenschaftliche Mitarbeiterin am Institut für Anatomie, Universitätsmedizin Rostock |
| 08/2022 | Promotion zum Dr. rer. hum. am Institut für Anatomie, Universitätsmedizin Rostock „Myelindegeneration als treibende Kraft der Immunzellrekrutierung: Myelinproteom, Schnittkulturen, Tiermodell“ |
| 12/2014 | Master of Science in Medizinischer Biotechnologie Abschlussarbeit: „Funktionelle Untersuchungen eines humanen neuronalen in vitro Modells des Morbus Niemann-Pick Typ C1 basierend auf differenzierten patientenspezifischen induzierten pluripotenten Stammzellen“ im Albrecht-Kossel-Institut für Neuroregeneration, Universitätsmedizin Rostock |
| 08/2014-06/2015 | Wissenschaftliche Hilfskraft im Albrecht-Kossel-Institut für Neuroregeneration, Universität Rostock |
| 07/2021 | Bachelor of Science in Medizinischer Biotechnologie Abschlussarbeit: „Die Osteoklastengenese im STR/ort-Mausmodell“ im Institut für Immunologie, Universitätsmedizin Rostock |
| 10/2011-01/2013 | Studentische Hilfskraft in Institut für Immunologie, Universität Rostock |
| 10/2009-12/2014 | Studium der Medizinischen Biotechnologie an der Universität Rostock |