AG Kaddatz
Our Interest
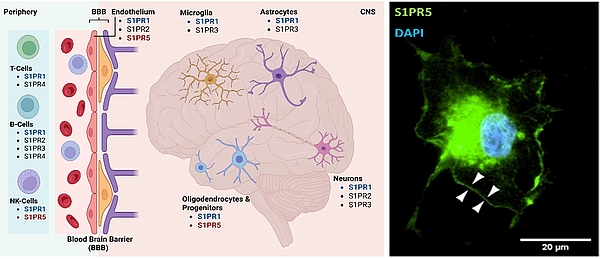
Sphingosine-1-phosphate-receptor modulation
The primary focus of our current research is on oligodendrocyte pathologies, the sphingosine-1-phosphate receptor (S1PR) system, and how this system is modulated by established pharmaceuticals, particularly Siponimod. We hypothesize that in diseases like multiple sclerosis, central cytoprotective effects of S1PR modulators, such as Siponimod, are mainly mediated through agonistic interactions with the S1PR5 receptor subtype. Oligodendrocytes, which express this receptor subtype at high levels, may be protected from cellular stress, degeneration, and demyelination as a result. Our central animal model for this research is the cuprizone model, which allows us to study pronounced oligodendrogliopathy, subsequent demyelination, glial cell activation, and immune cell recruitment in a non-autoimmune context. We use clinically established drugs (e.g., Siponimod) and various (conditional) mouse mutants in our investigations. The research is conducted on both murine and human brain tissue, employing established laboratory techniques such as paraffin and cryosectioning, immunohistochemistry, fluorescence microscopy, electron microscopy, real-time PCR and lipidomics.
Working on this project:
- cand. med. Emil Pril
- cand. med. dent. Justine Wallenta
- cand. med. Alina Peruth
- Dr. med. Hannes Kaddatz
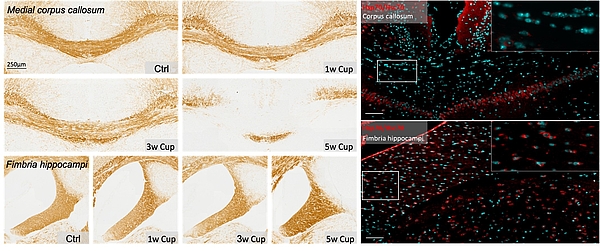
Regional oligodendrocyte vulnerability
Demyelination, characterized by oligodendrocyte loss and myelin damage, is a hallmark of neurological diseases like multiple sclerosis (MS). The cuprizone mouse model induces region-specific demyelination, affecting areas such as the corpus callosum and cortex, while regions like the commissura anterior, fimbria hippocampi and spinal cord remain largely unaffected ('pseudoresistant'). This study investigates regional differences in oligodendrocyte vulnerability, the role of cellular stress responses and the potential modulatory contribution of microglial CD83 expression.
Working on this project:
- cand. med. Sophia Meien
- Dr. med. Hannes Kaddatz
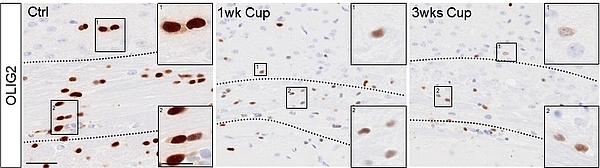
Loss of Oligodendrocyte Transcription Factors under Metabolic Stress
Oligodendrocyte transcription factor 2 (OLIG2) is a sequence-specific DNA-binding protein that plays a crucial role in oligodendrocyte lineage progression and early differentiation. While commonly used as a pan-oligodendrocyte marker, its stability and functional relevance under pathological conditions remain poorly understood. Recent in vitro studies indicate that oligodendrocyte lineage-enriched transcripts are particularly vulnerable to suppression during integrated stress responses, which may contribute to oligodendrocyte dysfunction and subsequent myelin degeneration. We hypothesize that OLIG2 protein expression in oligodendrocytes is dynamically regulated in response to cellular stress and that its loss may reflect an adaptive or maladaptive response. Here we test whether OLIG2 expression in oligodendrocytes is stable or altered during metabolic stress in vivo.
Working on this project:
- cand. med. Leo Heinig
- cand. med. Sophia Meien
- cand. med. Emil Pril
- Dr. med. Hannes Kaddatz
Cooperations
- Frank, Marcus; Medical Biology and Electron Microscopy Centre, Rostock University Medical Center, Germany
- Clarner, Tim; Anatomy and Cell Biology, Bonn University, Germany
- Dr. Gijs Kooij; Dept. Molecular Cell Biology & Immunology, Amsterdam Neuroscience & MS center Amsterdam, Location VUmc, Netherlands
- Dr. Luisa Müller, Institut für Transfusionsmedizin, Universitätsmedizin Greifswald, Germany
Most important publications
- Translocator protein is a marker of activated microglia in rodent models but not human neurodegenerative diseases. Nutma E, Fancy N, Weinert M, Tsartsalis S, Marzin MC, Muirhead RCJ, Falk I, Breur M, de Bruin J, Hollaus D, Pieterman R, Anink J, Story D, Chandran S, Tang J, Trolese MC, Saito T, Saido TC, Wiltshire KH, Beltran-Lobo P, Phillips A, Antel J, Healy L, Dorion MF, Galloway DA, Benoit RY, Amossé Q, Ceyzériat K, Badina AM, Kövari E, Bendotti C, Aronica E, Radulescu CI, Wong JH, Barron AM, Smith AM, Barnes SJ, Hampton DW, van der Valk P, Jacobson S, Howell OW, Baker D, Kipp M, Kaddatz H, Tournier BB, Millet P, Matthews PM, Moore CS, Amor S, Owen DR. Nat Commun. 2023 Aug 28;14(1):5247
- Transmembrane protein 119 is neither a specific nor a reliable marker for microglia. Vankriekelsvenne E, Chrzanowski U, Manzhula K, Greiner T, Wree A, Hawlitschka A, Llovera G, Zhan J, Joost S, Schmitz C, Ponsaerts P, Amor S, Nutma E, Kipp M, Kaddatz H. Glia. 2022 Jun;70(6):1170-1190
- Zhan J, Kipp M, Han W, Kaddatz H. Ectopic lymphoid follicles in progressive multiple sclerosis: From patients to animal models. Immunology. 2021 Nov;164(3):450-466.
- Cuprizone-induced demyelination triggers a CD8-pronounced T cell recruitment. Kaddatz H, Joost S, Nedelcu J, Chrzanowski U, Schmitz C, Gingele S, Gudi V, Stangel M, Zhan J, Santrau E, Greiner T, Frenz J, Müller-Hilke B, Müller M, Amor S, van der Valk P, Kipp M. Glia. 2021 Apr;69(4):925-942
Current and most recent funding
- Hertie-Stiftung - DrPrg SoSe 25 Kipp/Kaddatz/Pril Titel: Protektive Eigenschaften der Sphingosin-1-Phosphat Signalkaskade im Multiple Sklerose Tiermodell
- Hertie-Stiftung - DrPrg SoSe 26 Kipp/Kaddatz/Peruth Titel: Form und Funktion von Astrozyten in der Multiplen Sklerose: Die Rolle von Biomechanik und S1P-Signalweg
- „Oppenheim Förderpreis” for Multiple Sclerosis 2025 in the field of preclinical research by Novartis Pharma GmbH
- FORUN-Programm UMR Titel: Protective effects of the sphingosine-1-phosphate signaling cascade in neuroinflammation and degeneration